Class 9 SEBA Science Chapter 3 Solutions – Atoms and Molecules (2026–27) | Assam Eduverse
Chapter Overview:
SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions are prepared by Assam Eduverse strictly according to the latest SEBA / ASSEB syllabus 2026–27. These SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions are designed specifically for students searching for SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions that are accurate, updated, and fully exam-oriented. This page provides complete SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions, making it a reliable and trusted source for SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions based entirely on the official SEBA Class 9 Science textbook.
The SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions explain every concept included in SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions, such as laws of chemical combination, Dalton’s atomic theory, atoms, molecules, atomic mass, molecular mass, mole concept, and numerical problems. These SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions help students understand theory, definitions, numericals, and reasoning-based questions through SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions written in simple, clear, and exam-friendly language. Every SEBA Class 9 Science Chapter 3 Atoms and Molecules solution strictly follows the official ASSEB Class 9 Science Chapter 3 solutions format and marking scheme.
With the complete SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions (2026–27), students can confidently prepare SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions for intext questions as well as SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions for chapter-end textbook exercise questions. These SEBA Class 9 Science Chapter 3 Atoms and Molecules solutions include important Atoms and Molecules question answers, mole concept numericals, and exam-focused explanations. Assam Eduverse ensures every SEBA Class 9 Science Chapter 3 Atoms and Molecules solution is syllabus-based, conceptually accurate, and fully exam-focused for SEBA and ASSEB examinations.
SEBA / ASSEB Class 9 Science Chapter 3 – Atoms and Molecules Intext Questions & Answers (Latest Syllabus 2026–27)
📝 Page 32-33
Q1: In a reaction, 5.3 g of sodium carbonate reacted with 6 g of acetic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium acetate. Show that these observations are in agreement with the law of conservation of mass.
Answer: Given Data:
Reactants:
- Mass of sodium carbonate (Na₂CO₃) = 5.3 g
- Mass of acetic acid (CH₃COOH) = 6 g
- Total mass of reactants = 5.3 g + 6 g = 11.3 g
Products:
- Mass of carbon dioxide (CO₂) = 2.2 g
- Mass of water (H₂O) = 0.9 g
- Mass of sodium acetate (CH₃COONa) = 8.2 g
- Total mass of products = 2.2 g + 0.9 g + 8.2 g = 11.3 g
Conclusion:
Total mass of reactants = Total mass of products = 11.3 g
Hence, the observations are in agreement with the Law of Conservation of Mass, which states that:
“Mass can neither be created nor destroyed in a chemical reaction.”
Q2: Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Answer: Given that hydrogen and oxygen combine in the ratio 1:8 by mass to form water, this means 1 g of hydrogen requires 8 g of oxygen.
For 3 g of hydrogen:
Mass of oxygen required = 3 × 8 = 24 g
Therefore, 24 g of oxygen gas would be required to react completely with 3 g of hydrogen gas.
Q3: Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Answer: The postulate “Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction” is the result of the law of conservation of mass. This postulate explains that since atoms cannot be created or destroyed during chemical reactions, the total mass remains constant before and after the reaction.
Q4: Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Answer: The postulates “Atoms of a given element are identical in mass and chemical properties” and “Atoms combine in the ratio of small whole numbers to form compounds” together explain the law of definite proportions. Since atoms of the same element have identical mass and they combine in fixed ratios, compounds will always have the same elements in the same proportions by mass.
📝 Page 35
Q1: Define the atomic mass unit.
Answer: One atomic mass unit is a mass unit equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12. It is abbreviated as ‘u’ (unified mass) according to latest IUPAC recommendations. This unit is used to express the relative masses of atoms and molecules.
Q2: Why is it not possible to see an atom with naked eyes?
Answer: It is not possible to see an atom with naked eyes because atoms are extremely small in size. Their size is measured in nanometers (10⁻⁹ meters), and even millions of atoms stacked together would make a layer barely as thick as a sheet of paper. They are much smaller than the wavelength of visible light, making them impossible to see with naked eyes or even ordinary microscopes.
📝 Page 37
Q1: Write down the formulae of (i) sodium oxide (ii) aluminium chloride (iii) sodium sulphide (iv) magnesium hydroxide
Answer:
(i) Sodium oxide: Na₂O (ii) Aluminium chloride: AlCl₃ (iii) Sodium sulphide: Na₂S (iv) Magnesium hydroxide: Mg(OH)₂
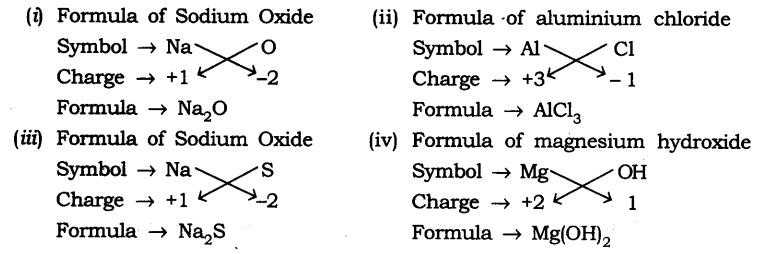
Q2: Write down the names of compounds represented by the following formulae: (i) Al₂(SO₄)₃ (ii) CaCl₂ (iii) K₂SO₄ (iv) KNO₃ (v) CaCO₃
Answer:
(i) Al₂(SO₄)₃ – Aluminium sulphate
(ii) CaCl₂ – Calcium chloride
(iii) K₂SO₄ – Potassium sulphate
(iv) KNO₃ – Potassium nitrate
(v) CaCO₃ – Calcium carbonate
Q3: What is meant by the term chemical formula?
Answer: A chemical formula is a symbolic representation of the composition of a compound. It shows the constituent elements present in the compound and the number of atoms of each element. The chemical formula uses symbols of elements and subscript numbers to indicate the ratio in which different atoms are present in the compound.
Q4: How many atoms are present in a (i) H₂S molecule and (ii) PO₄³⁻ ion?
Answer:
(i) H₂S molecule: Total atoms = 2 hydrogen atoms + 1 sulphur atom = 3 atoms
(ii) PO₄³⁻ ion: Total atoms = 1 phosphorus atom + 4 oxygen atoms = 5 atoms
📝 Page 40
Q1: Calculate the molecular masses of H₂, O₂, Cl₂, CO₂, CH₄, C₂H₆, C₂H₄, NH₃, CH₃OH.
Answer:
- H₂: 2 × 1 = 2 u
- O₂: 2 × 16 = 32 u
- Cl₂: 2 × 35.5 = 71 u
- CO₂: 12 + (2 × 16) = 12 + 32 = 44 u
- CH₄: 12 + (4 × 1) = 12 + 4 = 16 u
- C₂H₆: (2 × 12) + (6 × 1) = 24 + 6 = 30 u
- C₂H₄: (2 × 12) + (4 × 1) = 24 + 4 = 28 u
- NH₃: 14 + (3 × 1) = 14 + 3 = 17 u
- CH₃OH: 12 + (4 × 1) + 16 = 12 + 4 + 16 = 32 u
Q2: Calculate the formula unit masses of ZnO, Na₂O, K₂CO₃, given atomic masses of Zn = 65 u, Na = 23 u, K = 39 u, C = 12 u, and O = 16 u.
Answer:
- ZnO: 65 + 16 = 81 u
- Na₂O: (2 × 23) + 16 = 46 + 16 = 62 u
- K₂CO₃: (2 × 39) + 12 + (3 × 16) = 78 + 12 + 48 = 138 u
📝 Page 42
Question 1. If one mole of carbon atoms weigh 12 grams, what is the mass (in grams) of 1 atom of carbon?
Answer: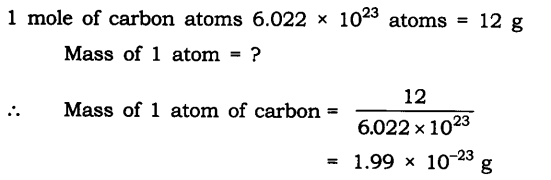
Question 2. Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given atomic mass of Na = 23 u, Fe = 56 u)?
Answer: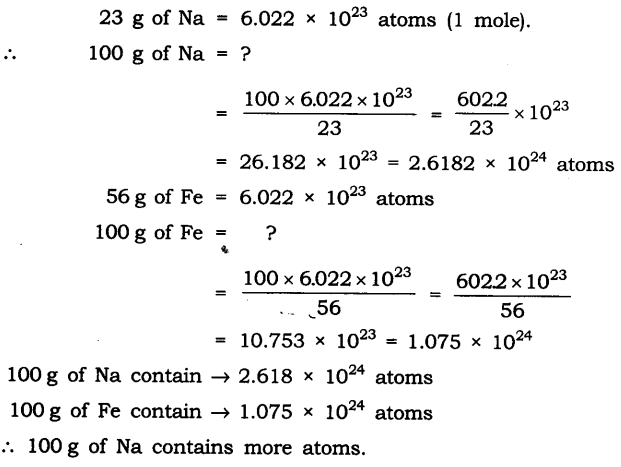
SEBA Class 9 Science Chapter 3 – Atoms and Molecules Textbook Exercise Questions & Solutions | 2026–27
📝 Page 43-44
Q1: A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Answer:
Total mass of compound = 0.24 g
Mass of boron = 0.096 g
Mass of oxygen = 0.144 g
Percentage of boron = (0.096/0.24) × 100 = 40%
Percentage of oxygen = (0.144/0.24) × 100 = 60%
Therefore, the percentage composition is 40% boron and 60% oxygen by weight.
Q2: When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
Answer: From the first reaction: 3.0 g carbon + 8.00 g oxygen → 11.00 g CO₂
This shows that 3.0 g of carbon requires only 8.00 g of oxygen to form 11.00 g of CO₂. In the second case, even though 50.00 g of oxygen is available, only 8.00 g will be consumed because that’s what 3.00 g of carbon requires.
Therefore, the same amount of carbon dioxide (11.00 g) will be formed, and 42.00 g of oxygen will remain unreacted.
This follows the Law of Definite Proportions (Law of Constant Proportions), which states that a pure compound always contains the same elements in the same proportions by mass.
Q3: What are polyatomic ions? Give examples.
Answer: Polyatomic ions are groups of atoms that carry a net electrical charge and behave as a single unit in chemical reactions. These ions contain more than one atom bonded together covalently, but the entire group has gained or lost electrons to acquire a charge.
Examples of polyatomic ions:
- Ammonium ion (NH₄⁺) – positively charged
- Hydroxide ion (OH⁻) – negatively charged
- Nitrate ion (NO₃⁻) – negatively charged
- Carbonate ion (CO₃²⁻) – negatively charged
- Sulphate ion (SO₄²⁻) – negatively charged
- Phosphate ion (PO₄³⁻) – negatively charged
Q4: Write the chemical formulae of the following:
(a) Magnesium chloride (b) Calcium oxide (c) Copper nitrate (d) Aluminium chloride (e) Calcium carbonate
Answer:
(a) Magnesium chloride: MgCl₂
(b) Calcium oxide: CaO
(c) Copper nitrate: Cu(NO₃)₂
(d) Aluminium chloride: AlCl₃
(e) Calcium carbonate: CaCO₃
Q5: Give the names of the elements present in the following compounds:
(a) Quick lime (b) Hydrogen bromide (c) Baking powder (d) Potassium sulphate
Answer:
(a) Quick lime (CaO): Calcium and Oxygen
(b) Hydrogen bromide (HBr): Hydrogen and Bromine
(c) Baking powder (NaHCO₃): Sodium, Hydrogen, Carbon, and Oxygen
(d) Potassium sulphate (K₂SO₄): Potassium, Sulphur, and Oxygen
Q6: Calculate the molar mass of the following substances:
(a) Ethyne, C₂H₂ (b) Sulphur molecule, S₈ (c) Phosphorus molecule, P₄ (Atomic mass of P = 31) (d) Hydrochloric acid, HCl (e) Nitric acid, HNO₃
Answer:
(a) Ethyne (C₂H₂): (2 × 12) + (2 × 1) = 24 + 2 = 26 g/mol
(b) Sulphur molecule (S₈): 8 × 32 = 256 g/mol
(c) Phosphorus molecule (P₄): 4 × 31 = 124 g/mol
(d) Hydrochloric acid (HCl): 1 + 35.5 = 36.5 g/mol
(e) Nitric acid (HNO₃): 1 + 14 + (3 × 16) = 1 + 14 + 48 = 63 g/mol
Q7: What is the mass of (a) 1 mole of nitrogen atoms? (b) 4 moles of aluminium atoms (Atomic mass of aluminium = 27)? (c) 10 moles of sodium sulphite (Na₂SO₃)?
Answer:
(a) 1 mole of nitrogen atoms:
Atomic mass of N = 14 u, so 1 mole = 14 g
(b) 4 moles of aluminium atoms:
Atomic mass of Al = 27 u, so 4 moles = 4 × 27 = 108 g
(c) 10 moles of sodium sulphite (Na₂SO₃):
Molar mass of Na₂SO₃ = (2 × 23) + 32 + (3 × 16) = 46 + 32 + 48 = 126 g/mol
Mass of 10 moles = 10 × 126 = 1260 g
Q8: Convert into mole: (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide
Answer:
(a) 12 g of oxygen gas (O₂):
Molar mass of O₂ = 32 g/mol
Number of moles = 12/32 = 0.375 mol
(b) 20 g of water (H₂O):
Molar mass of H₂O = 18 g/mol
Number of moles = 20/18 = 1.11 mol
(c) 22 g of carbon dioxide (CO₂):
Molar mass of CO₂ = 44 g/mol
Number of moles = 22/44 = 0.5 mol
Q9: What is the mass of: (a) 0.2 mole of oxygen atoms? (b) 0.5 mole of water molecules?
Answer:
(a) 0.2 mole of oxygen atoms:
Atomic mass of O = 16 u
Mass = 0.2 × 16 = 3.2 g
(b) 0.5 mole of water molecules:
Molecular mass of H₂O = 18 u
Mass = 0.5 × 18 = 9 g
Q10: Calculate the number of molecules of sulphur (S₈) present in 16 g of solid sulphur.
Answer:
Molar mass of S₈ = 8 × 32 = 256 g/mol
Number of moles in 16 g = 16/256 = 0.0625 mol
Number of molecules = 0.0625 × 6.022 × 10²³ = 3.76 × 10²² molecules
Q11: Calculate the number of aluminium ions present in 0.051 g of aluminium oxide.
Answer:
Formula of aluminium oxide = Al₂O₃
Molar mass of Al₂O₃ = (2 × 27) + (3 × 16) = 54 + 48 = 102 g/mol
Number of moles of Al₂O₃ = 0.051/102 = 0.0005 mol
Since each Al₂O₃ unit contains 2 Al³⁺ ions:
Number of Al³⁺ ions = 0.0005 × 2 × 6.022 × 10²³ = 6.022 × 10²⁰ ions
🎓 About Assam Eduverse
Assam Eduverse is the best educational platform in Assam, offering SEBA, AHSEC (ASSEB), SCERT, CBSE, and Assam Board Solutions along with study materials, notes, and exam preparation guides to help students learn smarter and score higher.
Our expert-prepared answers and MCQs follow the latest Assam Board Syllabus and NCERT Syllabus. We make learning simple, accessible, and effective for all students preparing for board or competitive exams.📘 Visit Assam Eduverse for free Assam Board Solutions, notes, and Study Materials prepared by experts.
