Class 9 SEBA Science Chapter 4 Solutions – Structure of the Atom (2026–27) | Assam Eduverse
Chapter Overview:
SEBA Class 9 Science Chapter 4 Structure of the Atom solutions are prepared by Assam Eduverse strictly according to the latest SEBA / ASSEB syllabus 2026–27. These SEBA Class 9 Science Chapter 4 Structure of the Atom solutions are created specifically for students searching for SEBA Class 9 Science Chapter 4 Structure of the Atom solutions that are accurate, updated, and exam-oriented. This page provides complete SEBA Class 9 Science Chapter 4 Structure of the Atom solutions, making it a reliable and trusted source for SEBA Class 9 Science Chapter 4 Structure of the Atom solutions based entirely on the official SEBA Class 9 Science textbook.
The SEBA Class 9 Science Chapter 4 Structure of the Atom solutions explain every concept included in SEBA Class 9 Science Chapter 4 Structure of the Atom solutions, such as discovery of subatomic particles, electrons, protons, neutrons, atomic models, Thomson’s model, Rutherford’s model, Bohr’s model, electronic configuration, atomic number, and mass number. These SEBA Class 9 Science Chapter 4 Structure of the Atom solutions help students understand theory, definitions, numericals, and reasoning questions using SEBA Class 9 Science Chapter 4 Structure of the Atom solutions written in simple, clear, and exam-friendly language. Every SEBA Class 9 Science Chapter 4 Structure of the Atom solution strictly follows the official ASSEB Class 9 Science Chapter 4 solutions format and marking scheme.
With the complete SEBA Class 9 Science Chapter 4 Structure of the Atom solutions (2026–27), students can confidently prepare SEBA Class 9 Science Chapter 4 Structure of the Atom solutions for intext questions as well as SEBA Class 9 Science Chapter 4 Structure of the Atom solutions for chapter-end textbook exercise questions. These SEBA Class 9 Science Chapter 4 Structure of the Atom solutions include important Structure of the Atom question answers, electronic configuration notes, and exam-focused explanations. Assam Eduverse ensures every SEBA Class 9 Science Chapter 4 Structure of the Atom solution is syllabus-based, conceptually accurate, and fully exam-focused for SEBA and ASSEB examinations.
SEBA / ASSEB Class 9 Science Chapter 4 – Structure of the Atom Intext Questions & Answers (Latest Syllabus 2026–27)
📝Page 47
Q1. What are canal rays?
Answer:
Canal rays are positively charged radiations discovered by E. Goldstein in 1886 during gas discharge experiments. They led to the discovery of the proton, a sub-atomic particle with a charge equal in magnitude but opposite in sign to that of the electron.
Q2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer:
No, the atom will not carry any net charge because the charge of one electron (−1) is balanced by the charge of one proton (+1), making the atom electrically neutral.
📝Page 49
Q1. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer:
According to Thomson’s model, an atom consists of a positively charged sphere in which negatively charged electrons are embedded. The total positive charge is equal to the total negative charge, so the atom as a whole is electrically neutral.
Q2. On the basis of Rutherford’s model of an atom, which sub-atomic particle is present in the nucleus of an atom?
Answer:
According to Rutherford’s model, protons are present in the nucleus of an atom.
Q3. Draw a sketch of Bohr’s model of an atom with three shells.
Answer: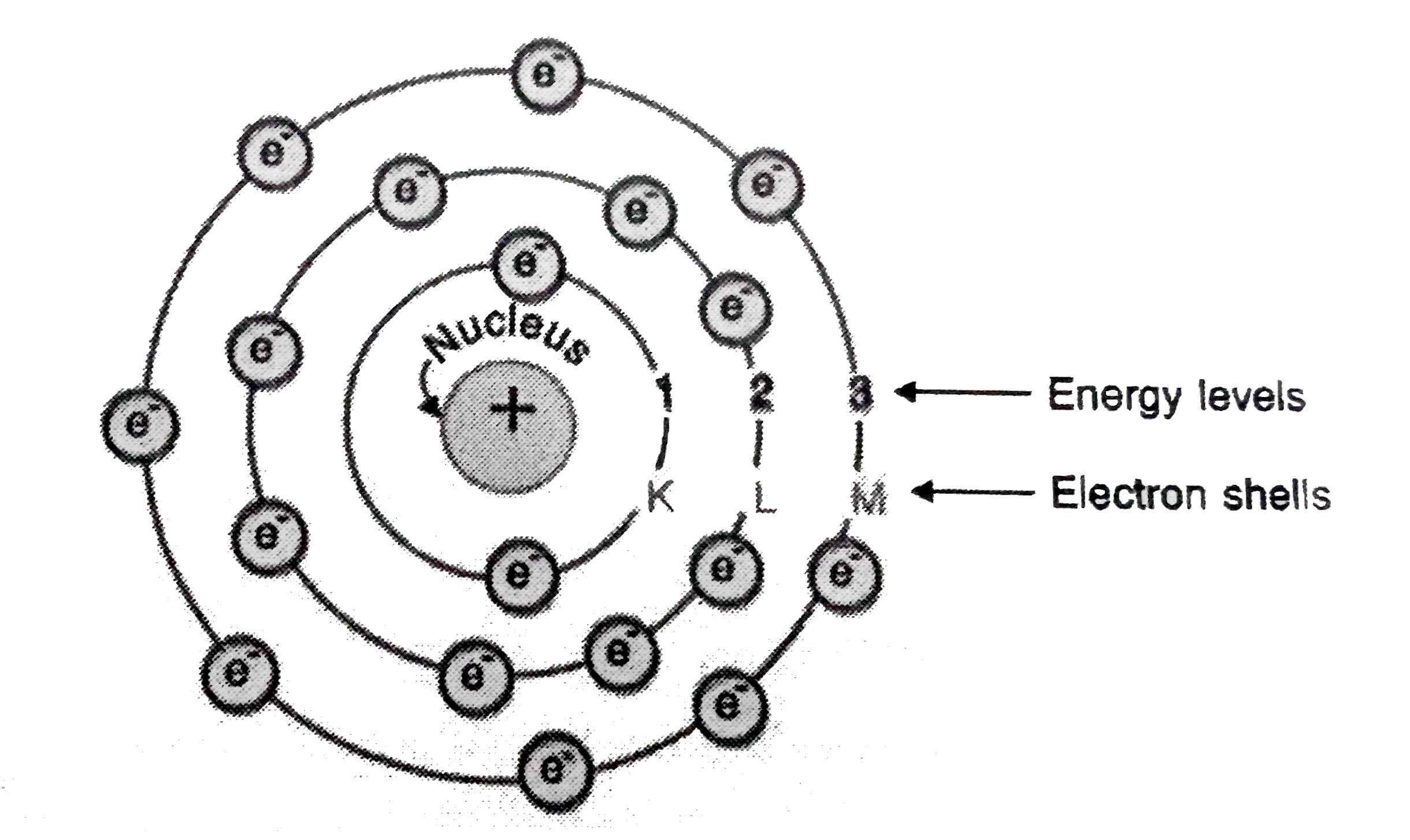
Q4. What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
Answer:
If a different metal foil is used, similar observations would be expected: most α-particles would pass through, a few would deflect at small angles, and a very few would bounce back. However, the degree of deflection might change depending on the atomic number and density of the metal used.
Q1. Name the three sub-atomic particles of an atom.
Answer:
The three sub-atomic particles are electrons, protons, and neutrons.
Q2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer:
Number of neutrons = Mass number − Number of protons
= 4 − 2 = 2
So, a helium atom has 2 neutrons.
📝Page 50
Q1. Write the distribution of electrons in carbon and sodium atoms.
Answer:
- Carbon (Atomic number = 6): Electronic configuration: 2, 4
- Sodium (Atomic number = 11): Electronic configuration: 2, 8, 1
Q2. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer:
K shell: maximum 2 electrons
L shell: maximum 8 electrons
Total = 2 + 8 = 10 electrons
📝Page 52
Q1. How will you find the valency of chlorine, sulphur and magnesium?
Answer:
- Chlorine: It has 7 electrons in its outermost shell, so valency = 8 − 7 = 1
- Sulphur: It has 6 electrons in its outermost shell, so valency = 8 − 6 = 2
- Magnesium: It has 2 electrons in its outermost shell, so valency = 2
Q1. If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom? and (ii) what is the charge on the atom?
Answer:
(i) Atomic number = number of protons = 8
(ii) The atom is electrically neutral because the number of protons (positive charges) equals the number of electrons (negative charges).
Q2. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Answer:
- Oxygen: Number of protons = 8, Number of neutrons = 8
Mass number = 8 + 8 = 16 - Sulphur: Number of protons = 16, Number of neutrons = 16
Mass number = 16 + 16 = 32
📝Page 53
Q1. For the symbol H, D and T, tabulate three sub-atomic particles found in each of them.
Answer:
Symbol | Protons | Neutrons | Electrons |
H (Protium) | 1 | 0 | 1 |
D (Deuterium) | 1 | 1 | 1 |
T (Tritium) | 1 | 2 | 1 |
Q2. Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
Example of Isotopes
Carbon-12 and Carbon-14
- Both have the same atomic number (Z = 6), so they have the same number of electrons and the same electronic configuration.
- Electronic configuration:
Carbon-12: 1s² 2s² 2p²
Carbon-14: 1s² 2s² 2p²
Example of Isobars
Calcium-40 and Argon-40
- They have the same mass number (A = 40) but different atomic numbers.
- Electronic configuration:
Calcium (Z = 20): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Argon (Z = 18): 1s² 2s² 2p⁶ 3s² 3p⁶
SEBA Class 9 Science Chapter 4 – Structure of the Atom Textbook Exercise Questions & Solutions | 2026–27
Q1. Compare the properties of electrons, protons and neutrons.
Answer: Properties of electrons, protons and neutrons.
Electron (e⁻)
- Negative charge (−1)
- Found outside the nucleus
- Very small mass (1/1836 of a proton)
- Controls chemical bonding and electricity flow
Proton (p⁺)
- Positive charge (+1)
- Found inside the nucleus
- Mass = 1 unit (standard)
- Determines the element’s atomic number
Neutron (n⁰)
- No charge (neutral)
- Found inside the nucleus
- Mass slightly more than a proton
- Contributes to atomic mass and nuclear stability
Q2. What are the limitations of J.J. Thomson’s model of the atom?
Answer:
Thomson’s model could not explain the results of the α-particle scattering experiment and did not provide any information about the arrangement of electrons inside the atom.
Q3. What are the limitations of Rutherford’s model of the atom?
Answer:
Rutherford’s model had some limitations. It could not explain the stability of the atom—moving electrons should lose energy and fall into the nucleus, but atoms remain stable. It also failed to explain the line spectra of elements, as it suggested a continuous spectrum. Lastly, it did not mention fixed energy levels for electrons, which Bohr later introduced.
Q4. Describe Bohr’s model of the atom.
Answer:
Bohr proposed that electrons revolve around the nucleus in fixed orbits or shells (energy levels) without radiating energy. Electrons only emit or absorb energy when they move between these shells. Each shell has a fixed energy.
Q5. Compare all the proposed models of an atom given in this chapter.
Answer:
The proposed models of an atom given in this chapter are-
- Thomson’s Model: Atom is a positively charged sphere with electrons embedded throughout, like a “plum pudding.”
- Rutherford’s Model: Atom mostly empty space; dense, positively charged nucleus at the center; electrons revolve around the nucleus.
- Bohr’s Model: Electrons revolve in fixed shells or orbits without losing energy; only certain orbits are allowed.
Q6. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer:
The rules for writing of distribution of electrons in various shells for the first eighteen elements are-
- Maximum electrons per shell = 2n², where n is the shell number (K=1, L=2, etc.)
- Maximum electrons in outermost shell is 8.
- Lower shells are filled first before outer ones.
Q7. Define valency by taking examples of silicon and oxygen.
Answer:
Valency is the combining capacity of an atom, determined by the number of electrons it gains, loses, or shares to complete its outer shell.
Silicon (Si) → Atomic number 14 → Electronic configuration: 2, 8, 4
- Has 4 electrons in its outer shell.
- Needs to share these 4 electrons to complete its octet.
- Valency = 4
Oxygen (O) → Atomic number 8 → Electronic configuration: 2, 6
- Has 6 electrons in its outer shell.
- Needs 2 more electrons to complete its octet.
- Valency = 2
Q8. Explain with examples (i) Atomic number, (ii) Mass number, (iii) Isotopes and (iv) Isobars. Give any two uses of isotopes.
Answer:
(i) Atomic number (Z) is the number of protons in the nucleus, e.g., Carbon (Z = 6).
(ii) Mass number (A) is the total number of protons and neutrons, e.g., Carbon (A = 12).
(iii) Isotopes are atoms of the same element with same atomic number but different mass numbers, e.g., ^12C and ^14C.
(iv) Isobars are atoms of different elements with different atomic numbers but same mass number, e.g., ^40Ar and ^40Ca.
Uses of isotopes are:
- Isotope of uranium (^235U) is used as fuel in nuclear reactors.
- Isotope of cobalt (^60Co) is used in cancer treatment.
Q9. Na has completely filled K and L shells. Explain.
Answer:
Sodium (Na) has atomic number 11. Its electronic configuration is 2 electrons in K, 8 in L and 1 in M shell. K and L shells are completely filled with their maximum capacities.
Q10. If bromine atom is available in the form of, say, two isotopes ^79Br (49.7%) and ^81Br (50.3%), calculate the average atomic mass of bromine atom.
Answer:
Average atomic mass = (79 × 49.7/100) + (81 × 50.3/100)
= (3936.3/100) + (4084.3/100)
= 39.363 + 40.843
= 80.206
So, the average atomic mass of bromine is 80.2 u (rounded to one decimal).
Q11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes X-16 and X-18 in the sample?
Answer:
Let % of X-16 = x, then % of X-18 = (100 − x)
So, 16.2 = (16x + 18(100 − x))/100
=> 16.2 = (16x + 1800 − 18x)/100
=> 16.2 = (1800 − 2x)/100
=> 1620 = 1800 − 2x
=> 2x = 1800 − 1620 = 180
=> x = 90
So, % of X-16 = 90%, X-18 = 10%
Q12. If Z = 3, what would be the valency of the element? Also, name the element.
Answer:
Z = 3 corresponds to lithium (Li). Configuration: 2, 1.
Valency = 1 (it can lose one electron to become stable).
Q13. Composition of the nuclei of two atomic species X and Y are given as under: X: Protons = 6, Neutrons = 6; Y: Protons = 6, Neutrons = 8.Give the mass numbers of X and Y. What is the relation between the two species?
Answer:
Mass number of X = 6 + 6 = 12
Mass number of Y = 6 + 8 = 14
Both have same atomic number but different mass numbers. They are isotopes of carbon.
Q14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about times that of a proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:
(a) False
(b) False
(c) True
(d)False
Q15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron
Answer: (a) Atomic Nucleus
Q16. Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers
Answer: (c) different number of neutrons
Q17. Number of valence electrons in Cl⁻ ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer: (b) 8
Q18. Which one of the following is a correct electronic configuration of sodium?
(a) 2,8
(b) 8,2,1
(c) 2,1,8
(d) 2,8,1
Answer: (d) 2,8,1
Q19. Complete the following table.
Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons | Name of the Atomic Species |
9 | 19 | 9 | 10 | 9 | Fluorine (F) |
16 | 32 | 16 | 16 | 16 | Sulphur (S) |
12 | 24 | 12 | 12 | 12 | Magnesium (Mg) |
🎓 About Assam Eduverse
Assam Eduverse is the best educational platform in Assam, offering SEBA, AHSEC (ASSEB), SCERT, CBSE, and Assam Board Solutions along with study materials, notes, and exam preparation guides to help students learn smarter and score higher.
Our expert-prepared answers and MCQs follow the latest Assam Board Syllabus and NCERT Syllabus. We make learning simple, accessible, and effective for all students preparing for board or competitive exams.📘 Visit Assam Eduverse for free Assam Board Solutions, notes, and Study Materials prepared by experts.
